1 when a hanging carpet is beaten with stick the dust particles start coming out of it because force of stick makes carpet to move to and fro but dust particles remain at rest due to inertia and hence separates out.
Mathematical formulation of first law of motion.
Actually is the other way round.
To formulate the laws of motion sir isaac newton treated massive bodies to be mathematical points.
This means that the object will continue moving with uniform velocity u throughout the time t.
For a thermodynamic process without transfer of matter the first law is often formulated displaystyle delta u q w where δu denotes the change in the internal energy of a closed system q denotes the quantity of energy supplied to the system as heat and w denotes the amount of thermodynamic work done by the system on its surroundings.
If no force is acting on a body its velocity does not change f 0 a 0.
The first law states that as object at rest will stay at rest and an object in motion will stay in motion unless acted on by a net external force mathematically this is equivalent to saying that if the net force on an object is zero then the velocity of the object is constant.
The first law is a particular case of the second law where f 0.
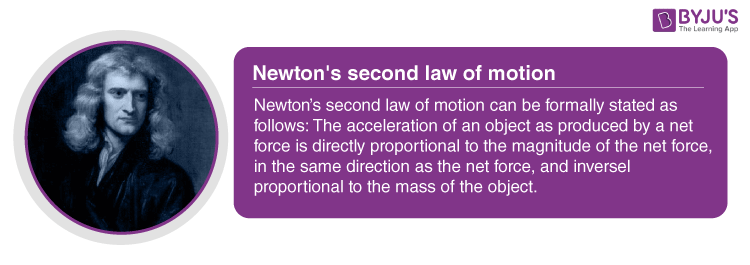
Essentially the second law is the mathematical formulation of the first one f ma f being the unbalanced force acting upon the other body.
Newtons s first law is called galileo s law of inertia.
Newton s first law is often referred to as the law of inertia.
Newton s first law formula newton s first law of motion is also referred to as the law of inertia.
That is when f 0 v u for whatever time t is taken.
In equation form the first law of thermodynamics is δu q w.
Newton s first law of motion states that an object at rest stays at rest and an object in motion stays in motion with the same speed and in the same direction unless acted upon by an unbalanced force.
Mathematical formulation of the first law of thermodynamics.
Similarly the energy of a system may be increased by doing work on the system in absence of heat e g by rubbing two objects together or passing electricity though a resistor.
The first law of thermodynamics states that the change in internal energy of a system equals the net heat transfer into the system minus the net work done by the system.
When heat is applied to a system the internal energy of the system will increase if no work is done.